Demonstrating better drug distribution for new bladder cancer treatment

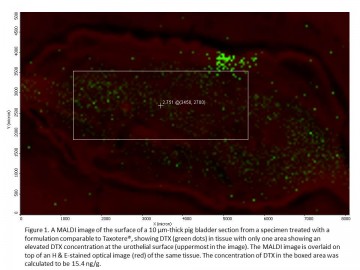
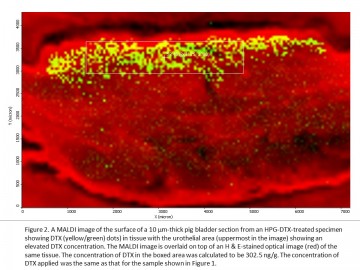
Current treatments for bladder cancer often fail because less than 1% of the chemotherapy drug is absorbed and stays in bladder tissues. Dr. David Plackett, a UBC pharmaceutical researcher and other members of the project team, partnered with UBC Imaging Labs to show that a promising new treatment gets into bladder tissues at a higher concentration than the commercial drug. By accessing the expert training, assistance and advice of UBC’s lab technicians and researchers, the team was able to use a MALDI mass spectrometer to provide compelling visual and quantitative evidence for an improved bladder cancer treatment. These results, combined with other studies on its effectiveness and safety, should give confidence to investors interested in funding the next stage of drug development. Dr. David Plackett, a Research Associate in the UBC Faculty of Pharmaceutical Sciences, has been working with the Centre for High Throughput Phenogenomics (CHTP), part of UBC Imaging Labs, to further advance the development of a new treatment for superficial bladder cancer. This promising new treatment has been developed by UBC Faculty of Pharmaceutical Sciences, The Centre for Drug Research and Development (CDRD), and CDRD Ventures Inc. (CVI), and funded in part by CIHR and Genome BC. It uses a chemically modified hyperbranched polyglycerol (HPG) as a drug carrier to achieve higher concentrations of the anti-cancer drug docetaxel (DTX) in the bladder wall than when this drug is administered alone in solution. A goal of this pre-clinical project has been to accurately measure the spatial distribution of DTX in bladder tissue and to compare it with the distribution of DTX when applied using a formulation comparable to the commercial anti-cancer drug Taxotere®. As an alternative to the established drug radiolabeling method for DTX analysis in bladder tissue and in collaboration with the project team, staff at CHTP have used the MALDI LTQ Orbitrap XL mass spectrometer from Thermo Scientific to both visualize DTX distribution in bladder tissue cross-sections and to quantify DTX across areas of tissue surface with nanogram sensitivity. A picture’s better than 1,000 words “MALDI imaging has the big advantage in that it gives us a visual picture of the drug distribution in bladder tissue that we don’t obtain from other methods. If we can show a significant difference in drug concentration visually between HPG-based formulations and commercial formulations such as Taxotere®, it will complement the other experimental data for publication and commercialization purposes,” says Dr. Plackett. There is an urgent need for new and more effective bladder cancer therapies in North America and globally. Chemotherapy often fails because the urothelial layer of the bladder forms a highly impermeable barrier to drugs, so that less than 1% of the drug instilled into the bladder is distributed into the bladder wall and then it is voided in one or two hours. The new HPG-DTX formulation to treat bladder cancer was developed by a team led by a number of UBC Principal Investigators in collaboration with the CDRD and its commercialization vehicle, CVI. The treatment is chemically designed to transiently increase the permeability of the bladder wall by attaching to the wall and causing temporary disruption of urothelial surface layer cells, known as umbrella cells, The aim of the project work with CHTP has been to use the powerful imaging capabilities of MALDI to provide a fuller perspective and confirmation of data obtained through the established analytical method using radiolabelled DTX. “We wanted to confirm in a visual way the differences in drug distribution and concentration of the new formulation for comparison with an often-used commercial formulation.” explains Dr. Plackett. “Furthermore, we’re able to overlay the MALDI image with a normal light microscopy view of the tissue section, along with a fluorescent image for the HPG, to yield an incredibly powerful view of how this new drug technology is working when applied to the urothelium.” Dedicated technical support for users The CHTP Lab has a team of technical staff with specialized expertise in operating the MALDI (matrix-assisted laser desorption/ionization) Mass Spectrometer and a wide variety of other imaging equipment to support industry and university researchers. “We have the experts on site that are 100% dedicated to supporting users. We offer full service expertise in mass spectrometry and our staff can carry out some or all of the steps from preparing the samples through to data analysis, based on the user’s needs. It’s a better experience for the user when you have an expert dedicated to helping you use the full capabilities of the equipment to get what you need,” says Dr. Nancy Ford, Director of the CHTP, one of UBC’s Imaging Labs. The research begins by treating ex vivo bladder tissue with the new HPG-DTX formulation and the commercial formulation. Bladder tissue samples are then supplied to a CHTP lab technician, who freezes them into blocks, followed by cryosectioning the tissue into thin (10 μm) sections on glass slides. Another CHTP lab technician receives the slides and coats them with a photoactive matrix, DHB, using a device called ImagePrep before the mass spectrometry analysis. At the same time, an adjacent section for each tissue sample is processed with H & E stain by Sun and each section is scanned using a Leica SP5 X Laser Scanning Confocal Microscope. After matrix coating, the slides are inserted into the MALDI LTQ Orbitrap XL mass spectrometer. In the mass spectrometer, the tissue sections are raster-scanned by laser with a spatial resolution of 50 μm, generating a mass spectrum for each measuring spot. The drug at each spot is then visualized using ImageQuest software provided by Thermo Scientific. After MALDI imaging measurement, the MALDI images can then be overlaid on top of histochemically stained images of adjacent tissue sections. “This allows for an improved visualization and understanding of drug distribution as a function of depth and tissue type within the treated bladder wall. The MALDI images have indicated a higher drug concentration in the urothelial layer of bladder samples when applying the HPG-DTX formulation,” says Dr. Plackett. Our lab technician’s expertise in MALDI mass spectrometry provided a detailed quantitative analysis of the concentrations of the new and commercial drug formulations at different tissue depths and locations to complement the qualitative analysis from visual mapping. A big advantage of MALDI imaging is that quantification of the drug can be done without loss of molecular spatial localization information in the tissue. The use of other common techniques for quantifying drugs, such as liquid chromatography-mass spectrometry (LC-MS) or gas chromatography-mass spectrometry (GC-MS), leads to a loss of information about localization of the drug in the tissue. Label-free imaging In drug distribution studies carried out using autoradiography, the drug is labeled with radioactive chemicals and only one compound can be analyzed in each experiment. Because MALDI imaging is label-free, it can be used to study drug distribution in the human body in clinical trials. As a label-free and multiplex technology, MALDI imaging also makes it possible to investigate a drug and its metabolites in one and the same measurement. Another advantage of the lab’s MALDI Orbitrap imaging system is that it has higher mass resolution and mass accuracy than the MALDI-TOF system in the field of low molecular weight compounds, such as pharmaceutical compounds. “The MALDI imaging system is sensitive to a range of ion or molecular sizes that are consistent with drug development. This can be a very useful tool for the drug and life science industries,” says Ford. Compelling visual evidence for investors The team’s work has provided a direct comparison between the new and commercial formulations, which has shown a higher concentration of DTX in surface layers of ex vivo bladder tissue when using HPG-DTX formulations. These results, obtained through MALDI imaging, provide compelling evidence for how the new treatment successfully overcomes the bladder wall’s permeability barrier and complements the efficacy and toxicology studies being carried out by other researchers in the project team. “To give confidence to investors interested in funding the next stage of drug development, an image is more powerful than data alone. This study provides both the quantification and visualization of drug and such findings can have significant value in moving us forward into the next stage of the project overall,” says Dr. Plackett
which form a natural impermeable barrier at the inner lining of the bladder. This allows for greater penetration of DTX into the bladder wall for an enhanced anti-cancer effect.